AAV Curator™ Platform

Andelyn's Suspension and Adherent Modular Platform Process
Optimization-By-Design™
Industrializing Gene Therapy Processes with a Reductionist, Holistic and Systematic Optimization Methodology Approach
Consistency in productivity and quality are evaluated at each stage of development and measured with enabling technologies.

Built on data and configurability, the platform ensures the end manufacturing process is curated to your program's specific needs to optimize yields.
Development of any platform process should account for optimization variables across upstream process, downstream, and fill-and-finish unit operations, materials, and quality control testing panels, as well as quality assurance release parameters.
Our modular approach uses configurable materials and methods optimized for your viral vector development and manufacturing requirements and reinforced with data and analytics.

- Adherent process: A low serum system utilizing a fortified calf serum (no FBS) with progressive reduction through the production process.
- Suspension Process: Utilizes chemically defined serum-free media for ultimate flexibility of scale across multiple bioreactor platforms, ensuring success in suspension culture and large-scale AAV vector production.
- Standard Bill of Materials including off-the-shelf (OTS) products that drive reduced lead times and security of supply, full QC testing.
AAV Curator® Cell Line
Clonal HEK293 cell line for high yield AAV production
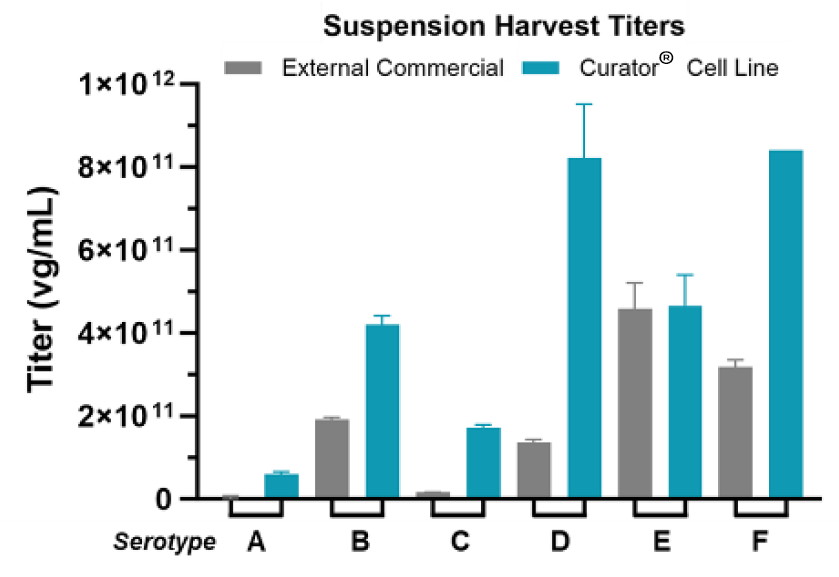
Andelyn's Curator® Cell Line is a clonal HEK293 cell line that improves rAAV titers across multiple serotypes.
- DoE was used to systematically improve rAAV titers for multiple serotypes
- Productivity assessed at harvest by collecting and testing supernatant
- Titers of vg/ml as determined by ddPCR
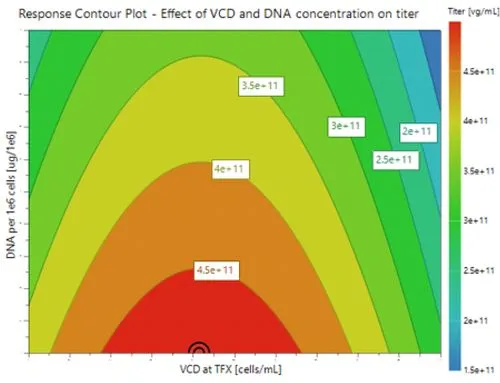
Optimal growth at high Viable Cell Density (VCD) to maximize viral vector production.
- The red region of the response contour plot indicates the design space which results in the highest overall yields of rAAV under the range of conditions investigated.
- Reduced DNA/cell in conjunction with optimal cell density to increase titer maximizes productivity.
Full Program Partnering

Upstream DoEs and downstream optimization can be done in parallel during feasibility and research productions
Benefits of Our Platform Approach
Reduced Spending on Optimization
High Productivity and % Full Capsids driving production costs down
No MCB Generation Costs
No Tech Transfer Costs
No PreClinical/clinical Licensing Fees
Reduced time spent on opTimization
Off the Shelf (OTS) plasmids readily avaIlable
No MCB generatIon time
Supply Chain Already Established
NO TECH TRANSFER/DOCUMENTATION TIME
DMFs filed for Andelyn’s platform
Same equipment in non-GMP & GMP
Same quality systems
Integrated teams in Preclinical & Clinical
Does your program use another vector rather than AAV?
Check out our Tech Transfer capabilities.